Hydrogen-Air Reaction Mechanism (CHEMKIN II/III)
Here an example from the CHEMKIN II/III user manual [1] is used to validate Chemked calculations against data obtained independently in another work. In the example [1], the chemistry of H2/O2/AR gas mixture was considered. The reaction mechanism of the mixture consists of 11 species and 23 reactions. A constant pressure problem is solved.
Input values
Pressure: 1atm
Temperature: 1000K
Mole fractions: [H2]0 : [O2]0 : [N2 ]0= 1 : 3 : 0.1
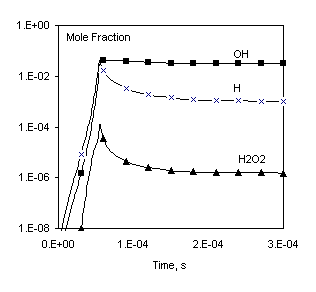
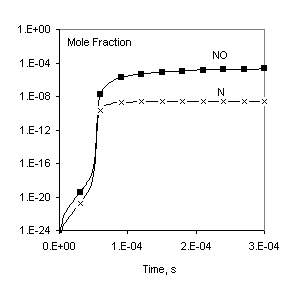
Fig. 1. Comparison of calculated profiles: points – CHEMKIN data [1], lines – Chemked calculation.
The same reaction mechanism and initial conditions are used to check calculation of equilibrium gas composition. In this case the problem must be solved to a long final time (for example, to 1 sec) to make sure that the equilibrium is established. These data are in excellent agreement with results obtained independently with the program CHEMKIN Equil.
References
[1] R.J. Kee, F.M. Rupley, J.A. Miller, M.E. Coltrin, J.F. Grcar, E. Meeks, H.K. Moffat, A.E. Lutz, G. Dixon-Lewis, M.D. Smooke, J. Warnatz, G.H. Evans, R.S. Larson, R.E. Mitchell, L.R. Petzold, W.C. Reynolds, M. Caracotsios, W.E. Stewart, P. Glarborg, C. Wang, and O. Adigun. CHEMKIN Collection, Release 3.6, Reaction Design, Inc., San Diego, CA (2000).